Abstract
Background : Immune Thrombocytopenia (ITP) is one of the most common acquired bleeding disorders affecting children, with an incidence rate of approximately 5-10 per 100,000 children per year. Intravenous immunoglobulin (IVIG) is an effective and widely employed frontline therapy for ITP; but is associated with substantial adverse effects, most commonly including headaches (~40%) and nausea/vomiting (5-26%). These may occur immediately following infusion or up to 72 hours later, often resulting in re-presentation to medical care. Additional diagnostic testing is often performed, including computed tomography (CT) imaging of the head, given concern for intracranial hemorrhage in these severely thrombocytopenic patients who have not yet responded to IVIG.
Objective : To identify rates of re-presentation to medical care due to IVIG-associated adverse effects within a cohort of treated pediatric ITP patients, and to identify the impact of this re-presentation to care on patient safety, health-related quality of life (HRQoL), and healthcare-related costs.
Methods : We completed a retrospective chart review, inclusive of all pediatric ITP patients receiving IVIG therapy at a large pediatric referral center within a 6-year timeframe from 2010 - 2016. Included patients were between the ages of 0 and 18 years, receiving IVIG for the primary indication of ITP at a dose of 1 g/kg/dose, and receiving the commercial IVIG product Gamunex-C® 10% (Grifols). All patients not meeting these criteria were excluded in order to maximize homogeneity of evaluated patients. Pre-medication with acetaminophen and diphenhydramine is the standard of practice for IVIG administration within the institution.
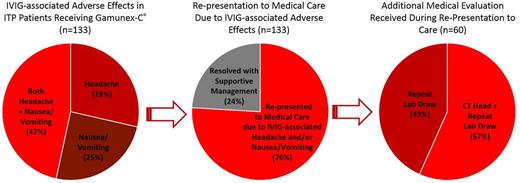
Results : Among the 473 ITP patients who received Gamunex-C® at Texas Children's Hospital from 2010 - 2016, 133 (28%) experienced documented headache, nausea/vomiting, or both following IVIG infusion, consistent with published incidences. Among these patients, 101 (76%) sought medical care due to these symptoms. Of the 81 patients who had already been discharged, 54 (67%) re-presented for Urgent or Emergency Care services; while 27 (33%) called for medical advice or to obtain supportive prescriptions. Of the 20 patients remaining hospitalized, 100% presented as calls to the on-call physician, resulting in additional medical evaluation, often including laboratory or imaging evaluation. Among all patients re-presenting to medical care, 60 (59%) underwent additional laboratory testing, and 36 (36%) underwent CT imaging to evaluate for life-threatening intracranial bleeding. No CT evaluations revealed evidence of intracranial bleeding, and for all 101 patients re-presenting to medical care, symptoms fully resolved with supportive management.
Conclusions : Nearly 1/3 of treated patients in our cohort experienced headache and/or nausea/vomiting, consistent with published incidences for Gamunex-C®. Of these, 76% sought further medical care. Subsequent medical management ranged from supportive care only (41%) to full evaluation with repeat laboratory studies (59%) + CT imaging in many cases (36%). These findings implicate substantially increased healthcare-related costs in the form of laboratory, CT imaging, and emergency service expenses; as well as increased risk to these patients' health and safety, in the form of additional radiation exposure, infection risk, and potential exposure to unnecessary therapies. Formal evaluation of downstream HRQoL and costs is ongoing. Additionally, we believe that a standardized prophylactic post-medication strategy could prevent or significantly reduce re-presentation to medical care due to IVIG-associated headache and/or nausea/vomiting, thereby improving HRQoL and cost impact in this patient population; and a formal prospective trial of this strategy is planned.
Lambert: AstraZeneca: Research Funding; Educational Concepts in Medicine: Honoraria; Novartis: Honoraria. Despotovic: Sanofi: Consultancy; Schell Cooley LLP: Other: Expert witness.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal